What is Crystal Engineering?
Crystal Engineering is a modern approach to solid state chemistry, where we exploit our understanding of intermolecular interactions (e.g. hydrogen bonds, π-stacking) to control the organization molecules in crystalline solids.
Effectively we use these intermolecular interactions do design and build unique crystal structures, which may demonstrate new properties or allow for solid state chemical reactions that would otherwise be unlikely or impossible in solution. Crystal engineering has led to the formation of multi-component structures composed of various building blocks and has emerged as a powerful approach to control the properties of solids. The modular nature crystal engineering allows for large compositional variation and provides a means to engineer properties based on the chemical nature and identities of the individual building blocks.
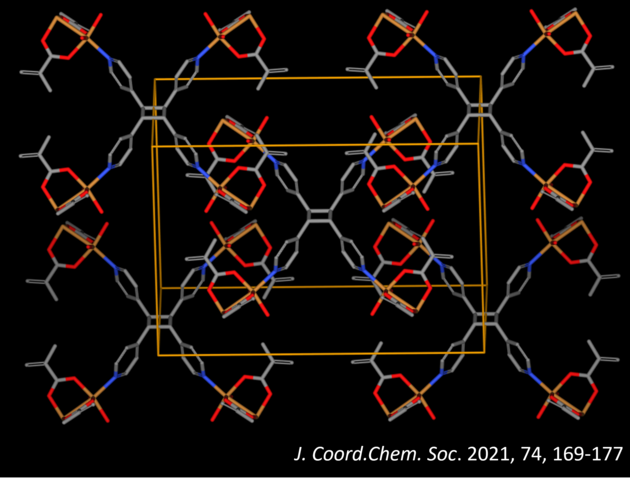
Some key examples of crystal engineering include the development of cocrystals and metal-organic frameworks (MOFs). Metal-organic materials are composed of a metal ion and single or multiple organic ligands that generally serve to bridge metal-ions in a solid. Cocrystals are crystalline materials composed of two or more different molecular or ionic partner components that are generally in a set ratio that arrange in space to create a unique crystal structure that differs from that of the individual building blocks.
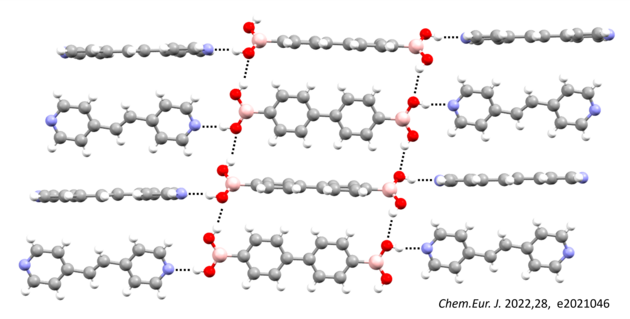
Careful crystal engineering allows us to use noncovalent interactions to obtain specific three-dimensional orientations of building blocks which is programmed into the system by the crystal packing. This provides us with a fine tune control over properties in three-dimensions such as optical, porosity, magnetic, solubility, stability, and conductivity. This fine tune control over molecular orientations and alignment also provides a method to reliably guide chemical reactions within the crystal. This allows organic crystals to be used as green chemical laboratories that allow for targeted construction of molecules and designer materials.
The method to control reactivity within organic crystals is green since traditional toxic organic solvents are minimized and generally not required for chemical reactions (i.e. solvent free), the reactions are atom economical (i.e. all reactants transformed to products) and highly selective (i.e. no by-products), the energy used for the reactions is provided by sunlight (i.e. ultraviolet), and the components that guide the reactions are recyclable templates and catalysts (i.e. similar to biochemical enzymes). The chemistry is innovative since the geometric information of templates and crystals can create entirely new classes of chemical products, with some being just recently discovered naturally (e.g. lipid ladderanes).