Crystal Engineering for Green Chemistry
Crystal engineering lends itself to green chemistry practices since reactions carried out within a crystal limit the use of toxic organic solvents since they are generally not required for chemical reactions (i.e. solvent free), the reactions are atom economical (i.e. all reactants transformed to products) and highly selective (i.e. no by-products), the energy used for the reactions is provided by sunlight (i.e. ultraviolet), and the components that guide the reactions are recyclable templates and catalysts (i.e. similar to biochemical enzymes).
Each of these features comprise the 12 Principles of Green Chemistry. In our research we are also developing methods to carry out solid-state reaction through mechanochemical and thermochemical processes which may provide methods for large scale production of materials while continuing to limit the use of toxic solvents.
Our goal is to control chemical reactions in organic crystals. While reactions have been known to occur in crystals for over a century, the ability of the solid state to be used like the liquid phase to routinely synthesize organic molecules has been unrealized. This has been due to the lack of a general method able to assemble wide varieties of reactants to react in solids.
What this has meant is that the true capacity of organic crystals to generate varieties of molecules, and the many green chemistry benefits that come along, have remained generally unrealized.
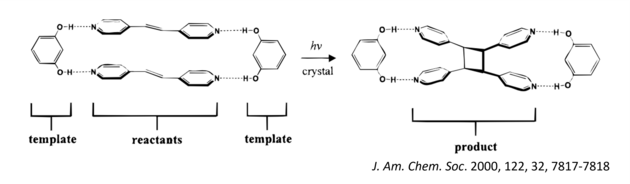
Our group has already shown that cocrystallization (i.e. the process of combining molecules) of 1,3-benzenediol, or resorcinol, with a bis(pyridyl)ethylene generates a multicomponent crystal composed of two different molecules, or a binary cocrystal, wherein the alkenes were positioned by hydrogen bonds to undergo a photodimerization reaction.
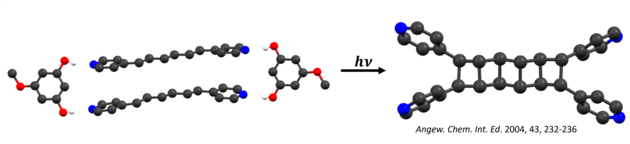
Irradiation of the cocrystal with ultraviolet (UV) light from either the laboratory or sun generated a tetra(pyridyl)cyclobutane (CB) as the sole product, in quantitative yield, and in gram amounts. The key to the method is that the template molecules isolated the reactants from effects of molecular packing. Furthermore, the template molecules can be easily recovered and recycled. Our group has further built on this method to produce architecturally and energetically rich CBs such as ladderanes and cubanes. The idea is to use organic crystals as green chemical laboratories that provide degrees of synthetic ‘freedom’ realized in solution.